Azithromycin (Zithromax): a semi-synthetic bacteriostatic antibiotic
Zithromax (Azithromycin) is a semi-synthetic bacteriostatic antibiotic with a broad spectrum of antimicrobial action, belongs to the group of macrolide-azalides. The substance is a derivative of erythromycin.
Mechanism of action
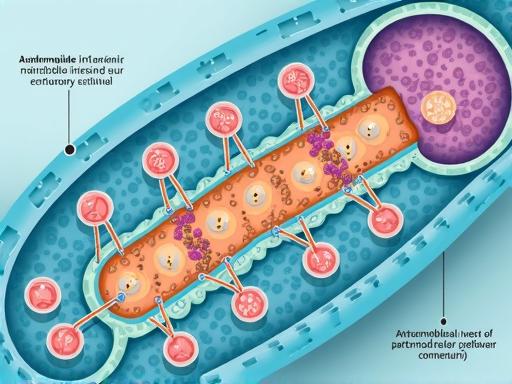
The action of the drug is based on the suppression of the synthesis of microbial cell protein, which results in a slowdown in the growth and reproduction of bacteria. The drug penetrates plasma membranes, making it effective against intracellular pathogens.
History and development
Azithromycin was first synthesized in 1980. The developer of the drug was the Croatian pharmaceutical company Pliva. Since 1988, the drug began to be released under the name “Sumamed”, over time, another name for the drug appeared on the market: “Zithromax”.
Antimicrobial spectrum
Azithromycin is active against a number of gram-positive, gram-negative, anaerobic, intracellular and other microorganisms, except for those resistant to erythromycin.
Among the microorganisms sensitive to the drug:
- Staphylococcus aureus,
- Streptococcus agalactiae,
- Pneumococcus,
- Pertussis bacillus,
- Haemophilus influenzae,
- Mycoplasma,
- Ureaplasma.
The drug is mainly used to treat respiratory diseases. Microorganisms may initially be resistant to the antibiotic (for example, Streptococcus pneumoniae is penicillin-resistant) or may acquire resistance to it.
Azithromycin is included in the standards of medical care for patients with pneumonia and chronic obstructive pulmonary disease, as well as in the “WHO Model List of Essential Medicines”.
Indications for use
- Infectious diseases of the upper respiratory tract and ENT organs (sinusitis, streptococcal pharyngitis/tonsillitis, otitis media);
- Infectious diseases of the lower respiratory tract (bacterial bronchitis, exacerbation of chronic bronchitis, interstitial and alveolar pneumonia);
- Infectious lesions of the skin and soft tissues (erysipelas, impetigo, secondarily infected dermatoses);
- Infectious diseases of the genitourinary system (urethritis, cervicitis);
- Lyme disease (borreliosis) in the initial stage (erythema migrans);
- Ulcers of the stomach and duodenum associated with Helicobacter pylori;
- Sexually transmitted infections (gonorrhea, chlamydia);
- Malaria.
Pharmacokinetics
The drug is resistant to acidic environments and dissolves well in fats. When taken orally, it is rapidly absorbed from the gastrointestinal tract. After a single dose of 500 mg, bioavailability is 37% (first-pass effect), the maximum concentration (0.4 mg/l) in the blood is achieved in 2-3 hours.
Effects on epithelial proteins
Azithromycin effects on proteins
In recent years, when studying the effects of azithromycin on proteins that form intercellular connections in the respiratory epithelium, new data have been obtained on the non-antibacterial properties of azithromycin, which are reversible and dose-dependent. It has been established that azithromycin increases the electrical resistance of the respiratory epithelium due to its effect on the regulation of ion and solution transport through the intercellular space and regulates the localization of epithelial intercellular contact proteins (claudin-1, claudin-4, adhesion molecule A), which ensure the integrity of the epithelial tissue and are a key component of the structural and functional protection of the respiratory tract epithelium. Other antibiotics do not have these properties.
Pediatric and respiratory infection data
For children suffering from bronchial asthma in combination with respiratory chlamydia.
According to the data, 62.5% of children with bronchial asthma have Chlamydophila pneumoniae infection of the respiratory tract, among them, the active course of infection is determined in 74.1%, latent – in 25.9% of children.
Moreover, for children with bronchial asthma in combination with respiratory chlamydia, a 3.8-fold decrease in the frequency of concomitant atopic diseases is characteristic compared to the data of patients not infected with Chlamydophila pneumoniae; 1.5-2.8 times – sensitization rates to food, household, pollen, epidermal, drug allergens and a 3-fold increase in the incidence of complicated forms of acute respiratory viral infections.
The inclusion of macrolides and interferons in the basic treatment of patients with bronchial asthma infected with Chlamydophila pneumoniae has increased the effectiveness of treatment measures (reducing the frequency and duration of exacerbations by 5 and 5.5 times, respectively, and the number of intercurrent diseases by 2.5 times).
COVID‑19: myths and evidence
Myths and Truths About the Use of Azithromycin (Zithromax) for COVID 19.
Early in the pandemic, there was some research and discussion about whether azithromycin could help treat COVID-19, especially when combined with other drugs such as hydroxychloroquine. However, most clinical trials have since shown that azithromycin does not have a significant effect on the outcome of COVID-19, especially if the patient does not have a bacterial infection such as pneumonia that requires antibiotics.
In some cases, azithromycin may be prescribed if a patient develops bacterial infections such as bacterial pneumonia in the context of COVID-19, but this decision is always made by the doctor.
Safety information
Contraindications: hypersensitivity to the drug, severe liver or kidney failure.
The antibiotic in the form of capsules and tablets is not prescribed to children under 12 years of age who have a body weight of less than 45 kg. For children over 6 months, the drug is prescribed in the form of a suspension for oral administration.
The use of azithromycin during pregnancy is possible in certain cases upon agreement with the doctor.
Among the side effects: gastrointestinal disorders (nausea, vomiting, diarrhea), as well as allergic reactions.

